"Entrepreneurs' aspiration" tells a story of their passion to start a business, the background and difficulties to overcome until the business gets on track, and their desires to realize through the business.
In the 38th installment, Mr. Morikuni Tobita, President and CEO of Gaudi Clinical Co., Ltd., talks with Tomoko Numata, the capitalist in charge of the company about future business challenges.
[Profile]
Morikuni Tobita, President of Gaudi Clinical Co., Ltd.
Graduating from a dental school, he joined the Japan Maritime Self-Defense Force. He engaged in clinical dental work at Self-Defense Forces hospitals at various bases, and then studied at a U.S. Navy hospital and a regenerative medicine bio-venture company, promoting research and development of regenerative medicine using adipose-derived stem cells. After transferring to Juntendo University, he was assigned and engaged in enforcing the Act on the Safety of Regenerative Medicine at the Ministry of Health, Labor and Welfare and in approval reviewing of regenerative medicine products at Pharmaceuticals and Medical Devices Agency (PMDA). Making use of these experiences, he contributed to accelerating implementation of medical technology in society, such as launching clinical trial support functions and support programs for practical application of seeds. In June 2021, with the establishment of Gaudi Clinical Co., Ltd., he assumed the position of President and Representative Director.
[What's Gaudi Clinical]
The company was founded in2021 with the mission of "connecting the last mile of regenerative medicine."
In the field of regenerative medicine which is attracting attention not only as a treatment for life-threatening diseases but also as a medical treatment aimed at improving quality of life(QOL), and on which universities and research institutes in Japan work more actively, the company engages in the contract cell manufacturing business, including comprehensive supports for medical institutions that perform regenerative medicine to deliver the safer, more trustful and reasonable technology of which validity is scientifically reviewed, from local medical institutions to their patients.
Connecting academia's regenerative medicine technology with medical institutions nationwide
ーCould you tell us about the current issues regarding regenerative medicine, which is the business domain of Gaudi Clinical?
Tobita In 2014, Act on the Safety of Regenerative Medicine (hereinafter referred to as the Safety Act) came into effect, establishing rules for the implementation of regenerative medicine as a medical treatment at one's own expense or a clinical research at medical institutions. However, whether the regenerative medicine provided is really effective and safe--we use the term "validity," is not yet reviewed to the point of verifying it.
Regenerative medicine involves the process of collecting patient cells, culturing them, and returning them to the patient's body. However, when some regenerative medicine, for example, a cell therapy given in the United States is applied to patients in Japan by doctors, whether the cell therapy is suitable for Japanese people is not properly verified by universities or research institutes. This is the current situation. In addition, it is quite difficult to conduct cell culture according to the rules in a medical institution, and outsourcing cell culture is becoming a recent trend, but not all the cultured cells are verified. Regenerative medicine is a new technology, so there are still many issues, and I think that is the biggest one.
ーWhat kind of regenerative medicine is the target of Gaudi Clinical 's business?
Tobita Regenerative medicine that improves quality of life, such as knee pain treatment and periodontal tissue regeneration. Regenerative medicine for underlying diseases and intractable diseases is developed as regenerative medicine products in accordance with Act on Pharmaceutical and Medical Devices (hereinafter referred to as the PMD Act). On the other hand, regenerative medicine that improves quality of life is generally provided as medical treatment at one's own expense in accordance with the above-mentioned Safety Act. Despite the fact that there are many people who need it, the current situation is that its validity is not confirmed enough and it is expensive, too.

ーHow will Gaudi Clinical solve the problem?
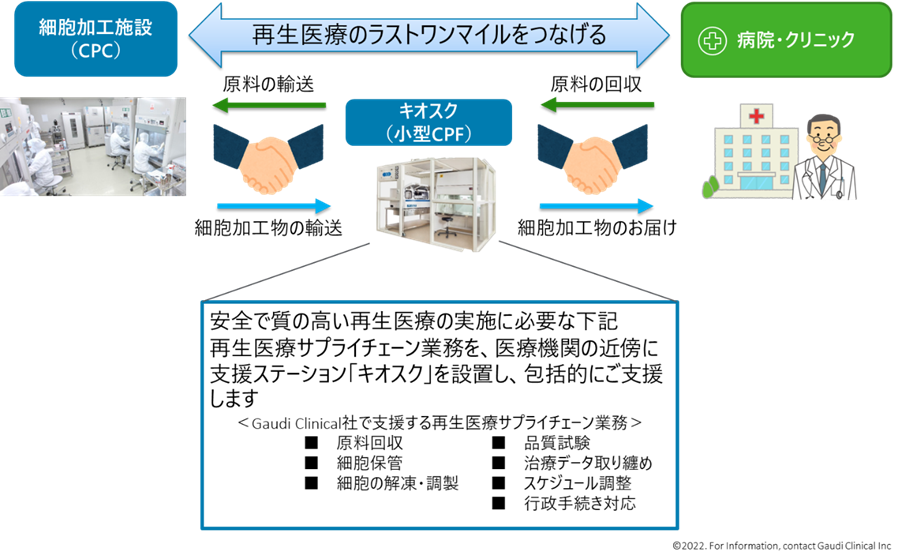
Tobita In Japan, many researchers are conducting research to confirm the safety and effectiveness of regenerative medicine. Therefore, we will contract with them for the technology that has been evaluated as valid, and will manufacture cells using that technology at the cell culture processing centers that we will set up nationwide. I would like to make the cells available at nearby medical institutions. This is an image of bridging to connect the technology of academia and medical institutions nationwide.
If we set up self-contained facilities that enable everything from raw material collection, transportation, manufacturing, storage, coordination, to support of acquiring treatment data nationwide and make them a platform, we can significantly reduce transportation costs to and from medical institutions. In addition, by setting up kiosk-type cell coordination rooms with regenerative medicine coordinators in various locations, , we can support normal activities of medical institutions such as preparing frozen cells before administration and collecting data on post-administration effects., By doing so, we believe that we will be able to provide more effective, safer, and cheaper regenerative medicine. When we launched our business, we conducted interviews with medical institutions and many answered that they wanted to provide regenerative medicine, but they weren't able to do so, which means there are needs.
ーMr. Tobita, please tell us how you decided to start a business in the field of regenerative medicine.
Tobita My first encounter with regenerative medicine was during my dental school days. I was shocked when I saw a photograph of a human ear reproduced on the back of a mouse in the scientific journal, Nature, and I strongly felt that I wanted to pursue a career in regenerative medicine. After graduating from the dental school, I joined the Maritime Self-Defense Force in order to earn income from clinical practice while conducting cutting-edge regenerative medicine research. I spent about half of a year aboard an escort ship providing medical treatment and humanitarian aid in times of disaster while also studying regenerative medicine when I was in Japan.
About 10 years after joining the Maritime Self-Defense Force, I began to consider practical applications of regenerative medicine, and entered Juntendo University. When I was about to start clinical research to confirm the validity of regenerative medicine which I researched in the Self-Defense Forces, I heard that the Safety Act is going to be enforced. The Safety Act is a very unique law in the world. In order to understand it, I thought that I would be better off experiencing the position of making rules, so although there was no recruitment, I negotiated directly and was assigned to the Ministry of Health, Labor and Welfare. I was also assigned to Pharmaceuticals and Medical Devices Agency PMDA, an administrative agency that reviews pharmaceuticals and others, and learned about the process of drug approval. Then I returned to the university and when I completed clinical research, I established a startup that would become Gaudi Clinical later.
- Do you feel that the experience you gained in the Self-Defense Forces and the government agencies is still useful today?
Tobita Before becoming a trainee, I first learned about leadership theory and organizational management at the Self-Defense Force. When I was dispatched to disaster areas overseas, I learned first-hand how to handle emergencies and crisis management. I feel that this is very useful in running a business. Also, at the Ministry of Health, Labor and Welfare and PMDA, I was able to learn everything necessary to commercialize what we learned from research and development, which enabled us to accelerate our subsequent research and development dramatically. We are now able to obtain large-scale research funds, and I believe that the experience has led to a big step towards commercialization.
Ms. Numata understood our unprecedented business model best
Mr. Tobita with JAFCO capitalist Tomoko Numata (left)
ーIn November 2022, you raised 360 million yen in the seed round through a lead investment by JAFCO. Please tell us about your first encounter with JAFCO.
Tobita My friend introduced me to Ms. Numata and I met her for the first time aroundJanuary2022. First of all, I took her to a cell culture processing center that we were contracted to operate by a company, and showed her the current situation. Our business model is unprecedented, so it is difficult to make it understood. On top of that, COVID-19 and Ukraine issues came up, which made it more difficult for us to raise funds. Under such circumstances, I've got to know Ms. Numata who truly understands our business and I am really appreciate it.
Numata Sure, you are going to develop a business utilizing regenerative medicine which is a new technology, and in accordance with the Safety Act, which requires specialized knowledge to invest in it. It can be said that it is a difficult area to evaluate for investors. However, what Gaudi Clinical is going to do is also a business in a sense that it has strong business evaluation axes, such as how to expand kiosk-type cell coordination rooms and how to reduce costs. That's what I felt when I heard his story, so I decided to seriously consider investing.
I am interested in industries with a strong sense of challenges, and have invested in healthcare-related companies, but this is my first time to invest in the regenerative medicine field. For example, when investing in a company that develops regenerative medicine products in accordance with the PMD Act, the criteria for evaluation have been established to some extent, and so investors who are accustomed to it would rather find it difficult to understand businesses such as Gaudi Clinical. So, I think it's good that I was able to face the business without having a fixed idea.

ーMr. Tobita, what was the decisive factor in choosing JAFCO as the lead investor? Ms. Numata, please tell us why you as a capitalist decided to invest in Gaudi Clinical.
Tobita Ms. Numata was by far the best in understanding our business. Therefore, I wanted to ask JAFCO from an early stage.
Numata Thank you . The reason why I decided to invest in Gaudi Cliical is first of all, there are not many entrepreneurs like Mr. Tobita. He joins the Self-Defense Forces to conduct a research on regenerative medicine, and transferred to the administrative agencies, which shows his strong energy to take the lead in jumping into the hottest areas. In addition, he proved that he is an excellent negotiator on every occasion when he needed to negotiate or coordinate with related organizations tactically in order to realize the project.
If it is realized, the project will be a profitable and socially significant business. I don't think anyone would be able to make it happen unless he takes on a challenge together with Mr. Tobita, who is a leading expert in regenerative medicine and an excellent entrepreneur.
Tobita I can't thank Ms. Numata enough. Many university-launched startups in Japan struggle to make an IPO, so we would like to repay JAFCO by achieving a successful IPO and becoming a role model for university-launched startups.
ー As a capitalist, what do you think about seed investment?
Numata JAFCO has been focusing on seed investments in recent years, and I would like to be actively involved in it if the opportunity arises. This time, I was able to be introduced to him at the best timing, but since many startups in the seed stage already have VCs, it is important to reach people who have ideas for startup as quickly as possible. I am constantly researching using SNS and so forth.

Delivering effective, safe, and inexpensive regenerative medicine throughout Japan
- Now that you have completed the fundraising, what are you planning to do in the future?
Tobita By 2027, we would like to establish cell processing centers and cell coordination rooms nationwide, and create a community with local medical institutions. To that end, we need doctors at medical institutions to understand our ideas and create the community together. Our company is a university-launched startup, and we have many employees with doctoral degrees and doctors who work as our technical advisors. I believe that being able to understand the position of medical institution-side is our strength in expanding the business.
-What kind of society do you want to realize through your business, Mr. Tobita?
Tobita First of all, I want to create a society where effective, safe, and inexpensive regenerative medicine can be delivered to as many people as possible. For example, if a parent or grandparent has a knee pain, and the child or grandchild wants to recommend regenerative medicine, saying, "There is good regenerative medicine, so let's try it," I think their affordable and the realistic price would be 300 to 400 thousand yen. But currently it costs several million yen, so I think it is very meaningful to change that point.

- Lastly, please tell us what you value as an entrepreneur.
Tobita The Self-Defense Force has the motto "medical readiness." I understand that it means "to be in full readiness," or in other words, "to be prepared," and I value that mindset as an entrepreneur.
Self-Defense Forces executives carry out their duties with the spirit of "being the first to land on a battlefield and the last to leave the battlefield." This attitude of demonstrating leadership with determination is essential in management of a company. If I, in spite of my position to lead the business, feel uneasy or timid, the doctors and the employees would become even more anxious. Therefore, I would like to maintain the attitude as we move forward with our business.
Office interior with motifs of the Ground, Maritime, and Air Self-Defense Forces
Comment from Person in charge: Tomoko Numata
 Prior to enactment of Act on the Safety of Regenerative Medicine (the Safety Act) in 2014, the market of medical treatment at one's own expense in regenerative medicine was relatively large. With the enactment of the Safety Act and establishment of rules for medical institutions to provide regenerative medicine, getting prompt and safe regenerative medicine treatment became possible while hurdles for the treatment providers got bigger, causing the market to shrink.. Gaudi Clinical 's business has the potential to recover the former market size through operational and pricing improvements. Regenerative medicine treatment that aims to improve QOL is expensive, but if the price drops, more people will be able to receive the treatment, which will change the way of regenerative medicine in Japan. I believe that the company, led by Mr. Tobita, who is not only a good researcher but an excellent manager, will become a leading company in the industry.
Prior to enactment of Act on the Safety of Regenerative Medicine (the Safety Act) in 2014, the market of medical treatment at one's own expense in regenerative medicine was relatively large. With the enactment of the Safety Act and establishment of rules for medical institutions to provide regenerative medicine, getting prompt and safe regenerative medicine treatment became possible while hurdles for the treatment providers got bigger, causing the market to shrink.. Gaudi Clinical 's business has the potential to recover the former market size through operational and pricing improvements. Regenerative medicine treatment that aims to improve QOL is expensive, but if the price drops, more people will be able to receive the treatment, which will change the way of regenerative medicine in Japan. I believe that the company, led by Mr. Tobita, who is not only a good researcher but an excellent manager, will become a leading company in the industry.





